- Get link
- X
- Other Apps








Advanced Pharmacognosy Notes
5,263 views
-
Be the first to comment
Advanced Pharmacognosy Notes
- 1. T.B.EKNATH BABU STUDENT AT ARULMIGU KALASALINGAM COLLEGE OF PHARMACY THIS ADVANCED PHARMACOGNOSY NOTES BELONGINGS TO Dr. TAMILNADU M.G.R MEDICAL UNIVERSITY TAMILNADU
- 2. TRACER TECHNIQUE
- 3. INTRODUCTION Living plants considered as biosynthetic laboratory as well as secondary metabolite. i) Different biosynthetic pathway: - Shikmic acid pathway Mevalonic acid pathway Acetate pathway ii) Various intermediate and steps are involved in biosynthetic pathway in plants can be investigated by means of following techniques: - Tracer technique Use of isolated organ Grafting methods Use of mutant strain
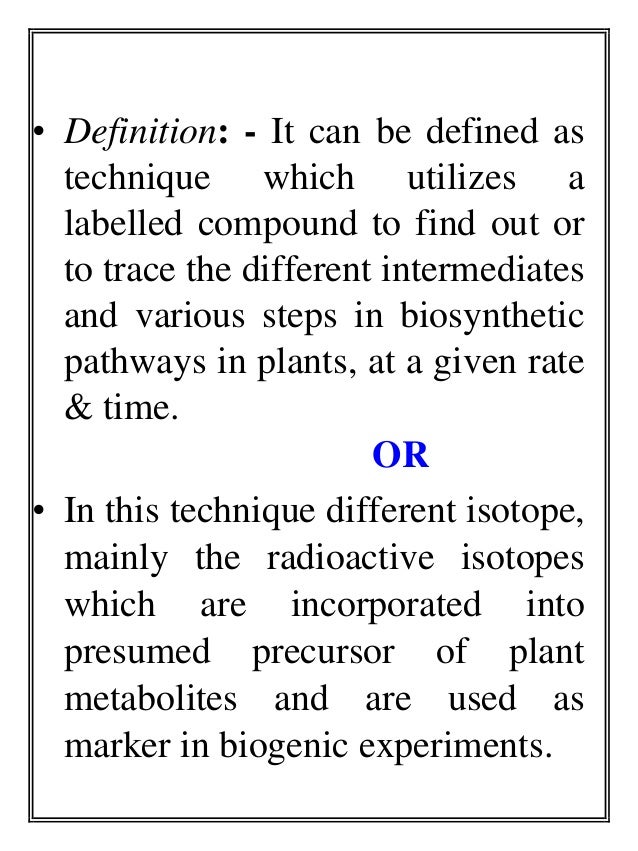
- 4. • Definition: - It can be defined as technique which utilizes a labelled compound to find out or to trace the different intermediates and various steps in biosynthetic pathways in plants, at a given rate & time. OR • In this technique different isotope, mainly the radioactive isotopes which are incorporated into presumed precursor of plant metabolites and are used as marker in biogenic experiments.
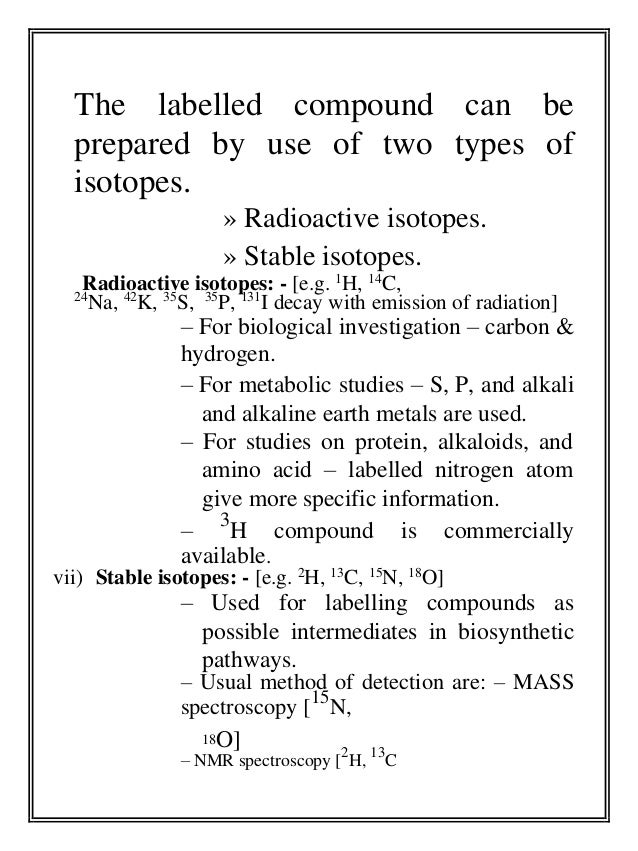
- 5. The labelled compound can be prepared by use of two types of isotopes. » Radioactive isotopes. » Stable isotopes. Radioactive isotopes: - [e.g. 1H, 14C, 24Na, 42K, 35S, 35P, 131I decay with emission of radiation] – For biological investigation – carbon & hydrogen. – For metabolic studies – S, P, and alkali and alkaline earth metals are used. – For studies on protein, alkaloids, and amino acid – labelled nitrogen atom give more specific information. 3 – H compound is commercially available. vii) Stable isotopes: - [e.g. 2H, 13C, 15N, 18O] – Used for labelling compounds as possible intermediates in biosynthetic pathways. – Usual method of detection are: – MASS spectroscopy [15N, 18O] 2 13 – NMR spectroscopy [ H, C
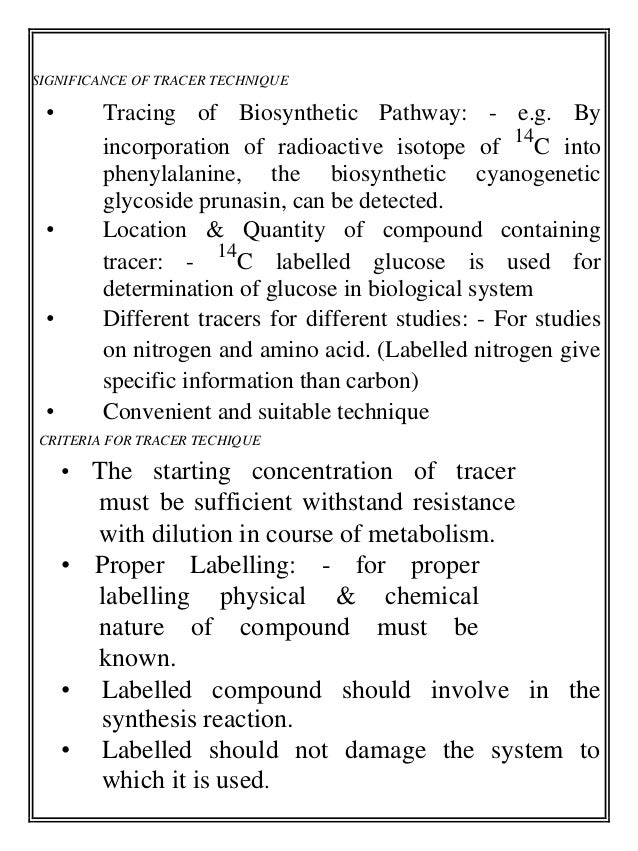
- 6. SIGNIFICANCE OF TRACER TECHNIQUE • Tracing of Biosynthetic Pathway: - e.g. By incorporation of radioactive isotope of 14 C into phenylalanine, the biosynthetic cyanogenetic glycoside prunasin, can be detected. • Location & Quantity of compound containing tracer: - 14 C labelled glucose is used for determination of glucose in biological system • Different tracers for different studies: - For studies on nitrogen and amino acid. (Labelled nitrogen give specific information than carbon) • Convenient and suitable technique CRITERIA FOR TRACER TECHIQUE • The starting concentration of tracer must be sufficient withstand resistance with dilution in course of metabolism. • Proper Labelling: - for proper labelling physical & chemical nature of compound must be known. • Labelled compound should involve in the synthesis reaction. • Labelled should not damage the system to which it is used.
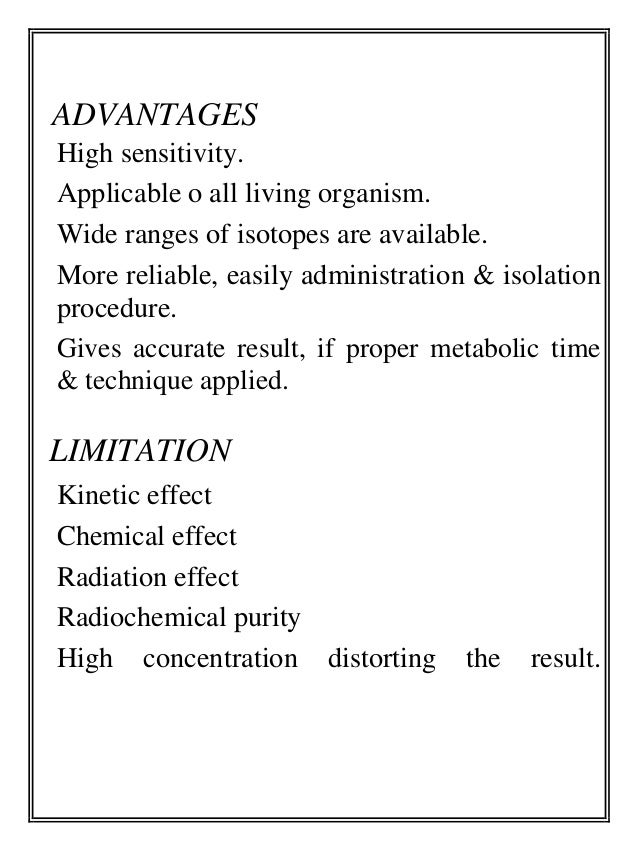
- 7. ADVANTAGES High sensitivity. Applicable o all living organism. Wide ranges of isotopes are available. More reliable, easily administration & isolation procedure. Gives accurate result, if proper metabolic time & technique applied. LIMITATION Kinetic effect Chemical effect Radiation effect Radiochemical purity High concentration distorting the result.
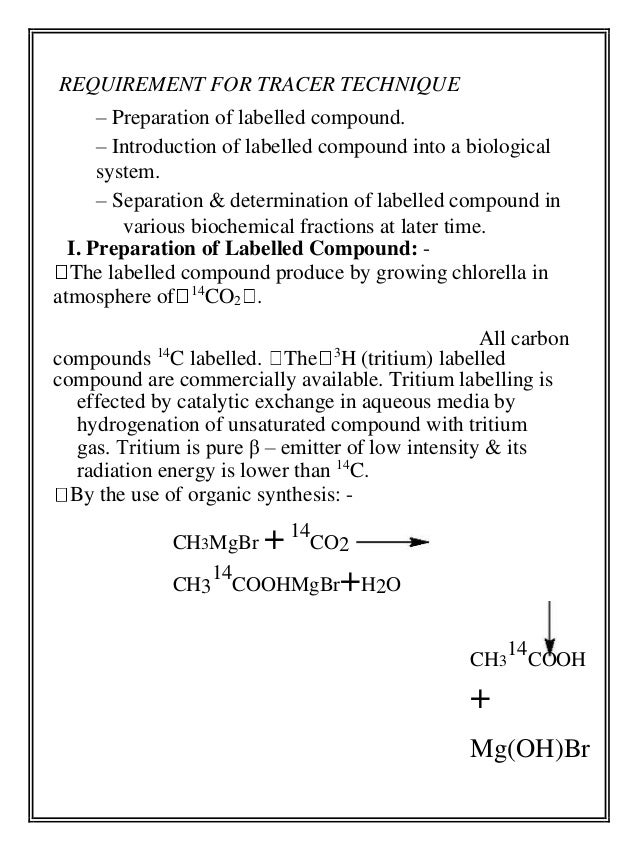
- 8. REQUIREMENT FOR TRACER TECHNIQUE – Preparation of labelled compound. – Introduction of labelled compound into a biological system. – Separation & determination of labelled compound in various biochemical fractions at later time. I. Preparation of Labelled Compound: - The labelled compound produce by growing chlorella in atmosphere of 14CO2 . All carbon compounds 14C labelled. The 3H (tritium) labelled compound are commercially available. Tritium labelling is effected by catalytic exchange in aqueous media by hydrogenation of unsaturated compound with tritium gas. Tritium is pure β – emitter of low intensity & its radiation energy is lower than 14C. By the use of organic synthesis: - CH3MgBr + 14 CO2 14 COOHMgBr+H2O CH3 CH3 14 COOH + Mg(OH)Br
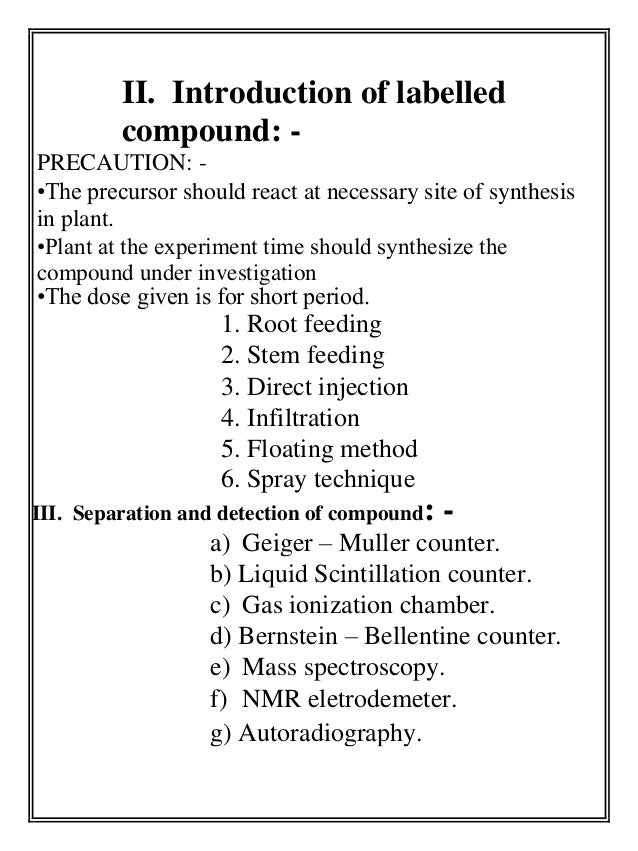
- 9. II. Introduction of labelled compound: - PRECAUTION: - •The precursor should react at necessary site of synthesis in plant. •Plant at the experiment time should synthesize the compound under investigation •The dose given is for short period. 1. Root feeding 2. Stem feeding 3. Direct injection 4. Infiltration 5. Floating method 6. Spray technique III. Separation and detection of compound: - a) Geiger – Muller counter. b) Liquid Scintillation counter. c) Gas ionization chamber. d) Bernstein – Bellentine counter. e) Mass spectroscopy. f) NMR eletrodemeter. g) Autoradiography.
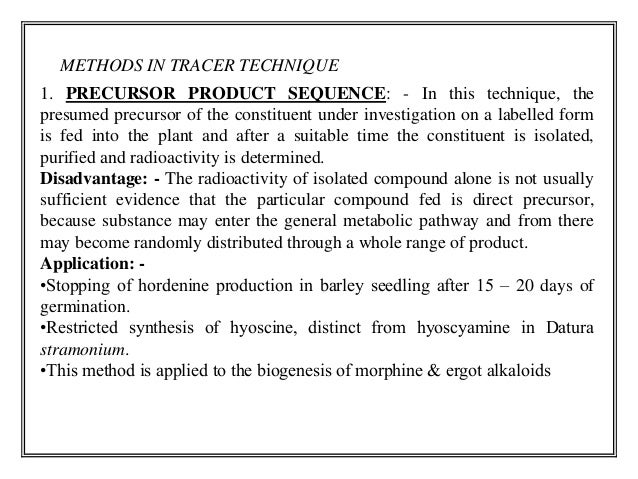
- 10. METHODS IN TRACER TECHNIQUE 1. PRECURSOR PRODUCT SEQUENCE: - In this technique, the presumed precursor of the constituent under investigation on a labelled form is fed into the plant and after a suitable time the constituent is isolated, purified and radioactivity is determined. Disadvantage: - The radioactivity of isolated compound alone is not usually sufficient evidence that the particular compound fed is direct precursor, because substance may enter the general metabolic pathway and from there may become randomly distributed through a whole range of product. Application: - •Stopping of hordenine production in barley seedling after 15 – 20 days of germination. •Restricted synthesis of hyoscine, distinct from hyoscyamine in Datura stramonium. •This method is applied to the biogenesis of morphine & ergot alkaloids
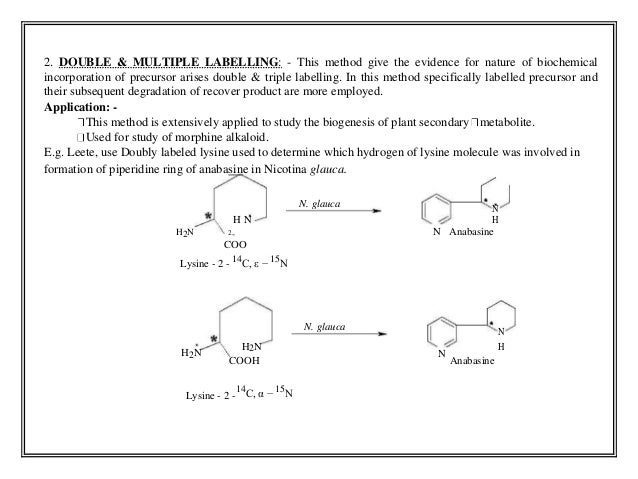
- 11. 2. DOUBLE & MULTIPLE LABELLING: - This method give the evidence for nature of biochemical incorporation of precursor arises double & triple labelling. In this method specifically labelled precursor and their subsequent degradation of recover product are more employed. Application: - This method is extensively applied to study the biogenesis of plant secondary metabolite. Used for study of morphine alkaloid. E.g. Leete, use Doubly labeled lysine used to determine which hydrogen of lysine molecule was involved in formation of piperidine ring of anabasine in Nicotina glauca. N. glauca N H N N H H2N 2- Anabasine COO Lysine - 2 - 14 C, ε − 15 Ν N. glauca N H2N H2N N H COOH Anabasine Lysine - 2 - 14 C, α − 15 Ν
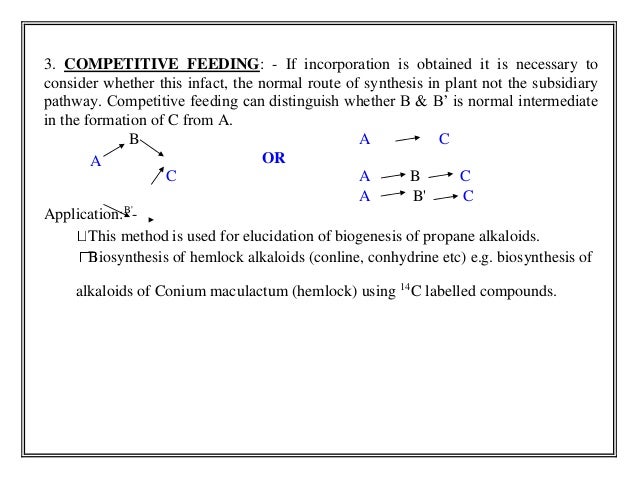
- 12. 3. COMPETITIVE FEEDING: - If incorporation is obtained it is necessary to consider whether this infact, the normal route of synthesis in plant not the subsidiary pathway. Competitive feeding can distinguish whether B & B’ is normal intermediate in the formation of C from A. B OR A C A C A B C Application:B'- A B' C This method is used for elucidation of biogenesis of propane alkaloids. Biosynthesis of hemlock alkaloids (conline, conhydrine etc) e.g. biosynthesis of alkaloids of Conium maculactum (hemlock) using 14C labelled compounds.
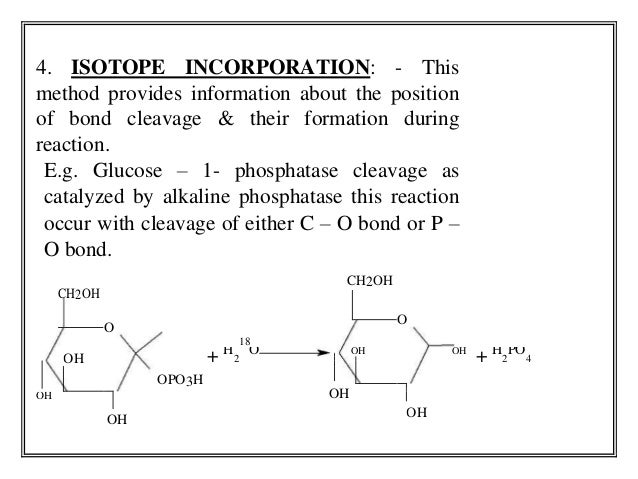
- 13. 4. ISOTOPE INCORPORATION: - This method provides information about the position of bond cleavage & their formation during reaction. E.g. Glucose – 1- phosphatase cleavage as catalyzed by alkaline phosphatase this reaction occur with cleavage of either C – O bond or P – O bond. CH2OH CH2OH O O 18 OH + H 2 O OH OH + H PO 2 4 OPO3H OH OH OH OH
- 14. 5. SEQUENTIAL ANALYSIS: - The principle of this method of investigation is to grow plant in atmosphere of 14CO 2 & then analyze the plant at given time interval to obtain the sequence in which various correlated compound become labelled. Application: - 14CO2 & sequential analysis has been very successfully used in elucidation of carbon in photosynthesis. Determination of sequential formation of opium hemlock and tobacco alkaloids. Exposure as less as 5 min. 14CO2, is used in detecting biosynthetic sequence as – Piperitone --------- (-) Menthone ------ ---- (-) Menthol in Mentha piperita.
- 15. APPLICATION OF TRACER TECHNIQUE 1. Study of squalene cyclization by use of 14C, 3H labelled mevalonic acid. 2. Interrelationship among 4 – methyl sterols & 4, 4 dimethyl sterols, by use of 14C acetate. 3. Terpenoid biosynthesis by chloroplast isolated in organic solvent, by use of 2- 14C mevalonate. 4. Study the formation of cinnamic acid in pathway of coumarin from labelled coumarin. 5. Origin of carbon & nitrogen atoms of purine ring system by use of 14C or 15N labelled precursor. 6. Study of formation of scopoletin by use of labelled phenylalanine. 7. By use of 45Ca as tracer, - found that the uptake of calcium by plants from the soil. (CaO & CaCO2). 8. By adding ammonium phosphate labelled with 32P of known specific activity the uptake of phosphorus is followed by measuring the radioactivity as label reaches first in lower part of plant, than the upper part i.e. branches, leaves etc.
- 16. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P PLANT TISSUE CULTURE TECHNIC 1. Introduction Plant tissue culture can be defined as the in vitro manipulation of plant cells and tissues and is a keystone in the foundation of plant biotechnology. It is useful for plant propagation and in the study of plant growth regulators. It is generally required to manipulate and regenerate transgenic plants. Whole plants can be regenerated under in vitro conditions using plant organs, tissues or single cells, by inoculating them in an appropriate nutrient medium under sterile environment. Plant tissue culture relies on the fact that many plant cells have the capacity to regenerate into a whole plant–a phenomena known as totipotency. Plant cells, cells without cell walls (protoplasts), leaves, or roots can be used to generate a new plant on culture media containing the necessary nutrients and plant growth regulators. Plant tissue culture was first attempted by Haberlandt (1902). He grew palisade cells from leaves of various plants but they did not divide. In 1934, White generated continuously growing cultures of meristematic cells of tomato on medium containing salts, yeast extract and sucrose and vitamin B (pyridoxine, thiamine and nicotinic acid) and established the importance of additives. In 1953, Miller and Skoog, University of Wisconsin – Madison discovered Kinetin, a cytokine that plays an active role in organogenesis. Plant cell cultures are an attractive alternative source to whole plants for the production of high-value secondary metabolites. 2. Advantages of plant tissue culture over conventional agricultural production The most important advantage of in vitro grown plants is that it is independent of geographical variations, seasonal variations and also environmental factors. It offers a defined production system, continuous supply of products with uniform quality and yield. Novel compounds which are not generally found in the parent plants can be produced in the in vitro grown plants through plant tissue culture. In addition, stereo- and region- specific biotransformation of the plant cells can be performed for the production of bioactive compounds from economical precursors. It is also independent of any political interference. Efficient downstream recovery of products and rapidity of production are its added advantages (Figure 31.1). ADVANCED PHARMACOGNOSY
- 17. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P Figure 31.1: Steps involved in the production of secondary metabolites from plant cell 3. Plant secondary metabolites Plant products can be classified into primary plant metabolites and secondary metabolites. Primary plant metabolites are essential for the survival of the plant. It consists of sugars, amino acids and nucleotides synthesized by plants and are used to produce essential polymers. Typically primary metabolites are found in all species within broad phylogenetic groupings, and are produced using the same metabolic pathway. Secondary metabolites are the chemicals, which are not directly involved in the normal growth and development, or reproduction of an organism. Secondary metabolites are not indispensable for the plants but play a significant role in plant defense mechanisms. Primary metabolites essentially provide the basis for normal growth and reproduction, while secondary metabolites for adaptation and interaction with the environment. The economic importance of secondary metabolites lies in the fact that they can be used as sources of industrially important natural products like colours, insecticides, antimicrobials, fragrances and therapeutics. Therefore, plant tissue culture is being potentially used as an alternative for plant secondary metabolite production. Majority of the plant secondary metabolites of interest to humankind fit into categories which categorize secondary metabolites based on their biosynthetic origin. Secondary metabolism in plants is activated only in particular stages of growth and development or during periods of stress, limitation of nutrients or attack by micro-organisms. Plants produce several bioactive compounds that are of importance in the healthcare, food, flavor and cosmetics industries. Many pharmaceuticals are produced from the plant secondary metabolites. Currently, many natural products are produced solely from massive quantities of whole plant parts. The source plants are cultured in tropical, subtropical, geographically remote areas, which are subject to drought, disease and changing land use patterns and other environmental factors. Secondary metabolites can be derived from primary metabolites through modifications, like methylation, hydroxylation and glycosylation. Secondary metabolites are naturally more complex than primary metabolites and are classified on the basis of chemical structure (e.g., aromatic rings, sugar), composition ADVANCED PHARMACOGNOSY
- 18. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P (containing nitrogen or not), their solubility in various solvents or the pathway by which they are synthesized (Table 31.1). They have been classified into terpenes (composed entirely of carbon and hydrogen), phenolics (composed of simple sugars, benzene rings, hydrogen and oxygen) and nitrogen and or sulphur containing compounds (Figure 31.2). It has been observed that each plant family, genus and species produces a characteristic mix of these bioactive compounds. All plants produce secondary metabolites, which are specific to an individual species, genus and are produced during specific environmental conditions which makes their extraction and purification difficult. As a result, commercially available secondary metabolites, for example, pharmaceuticals, flavours, fragrances and pesticides etc. are generally considered high value products as compared to primary metabolites and they are considered to be fine chemicals. Table 31.1: Classification of secondary metabolites Figure 31.2: The production of secondary metabolites is tightly associated with the pathways of primary/central metabolism, such as glycolysis, shikimate and production of aliphatic amino acids. 4. Strategies for enhanced production of secondary metabolites in plant cell cultures ADVANCED PHARMACOGNOSY
- 19. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P 4.1. Proper selection of cell lines The heterogeneity within the cell population can be screened by selecting cell lines capable of accumulating higher level of metabolites. 4.2. Manipulation of medium The constituents of culture medium, like nutrients, phytohormones and also the culture conditions, like temperature, light etc. influence the production of secondary metabolites. For e.g., if sucrose concentration is increased from 3% to 5%, production of rosamarinic acid is increased by five times. In case of shikonin production, IAA enhances the yield whereas 2,4-D and NAA are inhibitory. 4.3. Addition of Elicitors Elicitors are the compounds which induce the production and accumulation of secondary metabolites in plant cells. Elicitors produced within the plant cells include cell wall derived polysaccharides, like pectin, pectic acid, cellulose etc. Product accumulation also occurs under stress conditions caused by physical or chemical agents like UV, low or high temperature, antibiotics, salts of heavy metals, high salt concentrations which are grouped under abiotic elicitors. Addition of these elicitors to the medium in low concentration enhances the production of secondary metabolites. 4.4. Addition of precursors Precursors are the compounds, whether exogenous or endogenous, that can be converted by living system into useful compounds or secondary metabolites. It has been possible to enhance the biosynthesis of specific secondary metabolites by feeding precursors to cell cultures. For example, amino acids have been added to suspension culture media for production of tropane alkaloids, indole alkaloids. The amount of precursors is usually lower in callus and cell cultures than in differentiated tissues. Phenylalanine acts as a precursor of rosmarinic acid; addition of phenylalanine to Salvia officinalis suspension cultures stimulated the production of rosmarinic acid and decreased the production time as well. Phenylalanine also acts as precursor of the N-benzoylphenylisoserine side chain of taxol; supplementation of Taxus cuspidata cultures with phenylalanine resulted in increased yields of taxol. The timing of precursor addition is critical for an optimum effect. The effects of feedback inhibition must surely be considered when adding products of a metabolic pathway to cultured cells. 4.5. Permeabilisation Secondary metabolites produced in cells are often blocked in the vacuole. By manipulating the permeability of cell membrane, they can be secreted out to the media. Permeabilisation can be achieved by electric pulse, UV, pressure, sonication, heat, etc. Even charcoal can be added to medium to absorb secondary metabolites. 4.6. Immobilisation Cell cultures encapsulated in agarose and calcium alginate gels or entrapped in membranes are called immobilised plant cell cultures. Immobilization of plant cells allows better cell to cell contact and the cells are also protected from high shear stresses. These immobilized systems can effectively increase the productivity of secondary metabolites in a number of species. Elicitors can also be added to these systems to stimulate secondary metabolism. ADVANCED PHARMACOGNOSY
- 20. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P 4.7. Limitations • Production cost is often very high. • Lack of information of the biosynthetic pathways of many compounds is a major drawback in the improvement of their production. • Trained technical manpower is required to operate bioreactors. 5. Advantages of cell, tissue and organ cultures as sources of secondary metabolites 5.1. Plant cell cultures Once interesting bioactive compounds have been were identified from plant extracts, the first part of the work consisted in collecting the largest genetic pool of plant individuals that produce the corresponding bioactive substances. However, a major characteristic of secondary compounds is that their synthesis is highly inducible, therefore, it is not certain, if a given extract is a good indicator of the plant potential for producing the compounds. The ability of plant cell cultures to produce secondary metabolites came quite late in the history of in vitro techniques. For a long time, it was believed that undifferentiated cells, such as callus or cell suspension cultures were not able to produce secondary compounds, unlike differentiated cells or specialized organs. 5.2. Callus culture Callus is a mass of undifferentiated cells derived from plant tissues for use in biological research and biotechnology. In plant biology, callus cells are those cells that cover a plant wound. To induce callus development, plant tissues are surface sterilized and then plated onto in vitro tissue culture medium. Different plant growth regulators, such as auxins, cytokinins, and gibberellins, are supplemented into the medium to initiate callus formation. It is well known that callus can undergo somaclonal variations, usually during several subculture cycles. This is a critical period where, due to in vitro variations, production of secondary metabolite often varies from one subculture cycle to another. When genetic stability is reached, it is necessary to screen the different cell (callus) lines according to their aptitudes to provide an efficient secondary metabolite production. Hence, each callus must be assessed separately for its growth rate as well as intracellular and extracellular metabolite concentrations. This allows an evaluation of the productivity of each cell line so that only the best ones will be taken for further studies, for example, for production of the desired compound in suspensions cultures. 5.3. Cell suspension cultures Cell suspension cultures represent a good biological material for studying biosynthetic pathways. They allow the recovery of a large amount of cells from which enzymes can be easily separated. Compared to cell growth kinetics, which is usually an exponential curve, most secondary metabolites are often produced during the stationary phase. This lack of production of compounds during the early stages can be explained by carbon allocation mainly distributed for primary metabolism when growth is very active. On the other hand, when growth stops, carbon is no longer required in large quantities for primary metabolism and secondary compounds are more actively synthesized. However, some of the secondary plant products are known to be growth-associated with undifferentiated cells, such as betalains and carotenoids. 5.4. Organ cultures ADVANCED PHARMACOGNOSY
- 21. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P Plant organs are alternative to cell cultures for the production of plant secondary metabolites. Two types of organs are generally considered for this objective: hairy roots and shoot cultures. A schematic representation of various organized cultures, induced under in vitro conditions, is given in Figure 31.3. 5.4.1. Shoot cultures Shoots exhibit some comparable properties to hairy roots, genetic stability and good capacities for secondary metabolite production. They also provide the possibility of gaining a link between growth and the production of secondary compounds. 5.4.2. Hairy root cultures Hairy roots are obtained after the successful transformation of a plant with Agrobacterium rhizogenes. They have received considerable attention of plant biotechnologists, for the production of secondary compounds. They can be subcultured and indefinitely propagated on a synthetic medium without phytohormones and usually display interesting growth capacities owing to the profusion of lateral roots. This growth can be assimilated to an exponential model, when the number of generations of lateral roots becomes large. Cell Suspension culture ADVANCED PHARMACOGNOSY
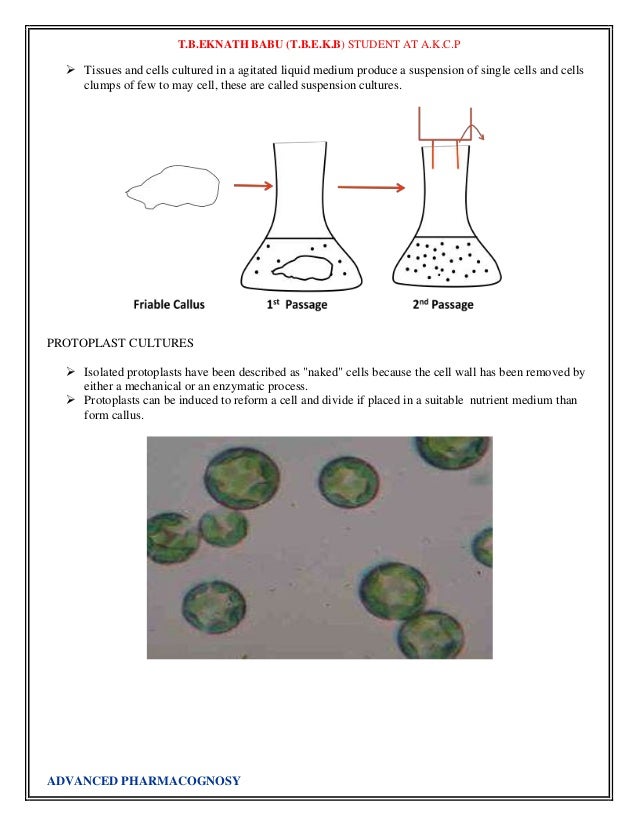
- 22. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P Tissues and cells cultured in a agitated liquid medium produce a suspension of single cells and cells clumps of few to may cell, these are called suspension cultures. PROTOPLAST CULTURES Isolated protoplasts have been described as "naked" cells because the cell wall has been removed by either a mechanical or an enzymatic process. Protoplasts can be induced to reform a cell and divide if placed in a suitable nutrient medium than form callus. ADVANCED PHARMACOGNOSY
- 23. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P ADVANCED PHARMACOGNOSY
- 24. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P Ovary/ovule culture Ovary or ovule culture involves development of haploid from unfertilized cells of embryosac present in ovary. ADVANCED PHARMACOGNOSY
- 25. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P CELLULAR TOTIPOTENCY In the preceding units of this course you have read that innumerable cells which constitute the body of a higher plant or animal and containing identical genetic material can be traced to a single cell-the zygote. During development cells undergo diverse structural and functional specialisation depending upon their position in the body. Leaf cells bear chloroplasts and act as the site of photosynthesis. The colourless root hairs perform the function of absorbing nutrients and water from the soil and some other cells become part of the colourful petals. Normally fully differentiated cells do not revert back to a meristematic: state, which suggests that the cells have undergone a permanent change. In earlier sections of this unit you have read that the regenerative capacity is retained by all living cells of a plant. Several horticultural plants regenerate whole plant from root, leafiand stem cuttings. Highly differentiated and mature cells such as those of pith and cortex and highly specialised cells as those of microspores and endosperm,retain full potential to give rise to full plants under suitable culture conditions. G. Haberlandt was the first to test this idea experimentally. This endowment called "cellular totipotency" is unique to plants. Animal cells possibly because of their higher degree of specialisation do not exhibit totipotency. Whole plant regeneration from cultured cells may occur in one of the two pathways: ;)shoot bud differentiation, (organogenesis) and ii) embryo formation (Embryogenesis). The Embryos are bipolar structures with no organic connection with the parent tissue and can germinate directly into a complete plant. On the other hand, shoots are monopolar. They need to be removed from the parent tissue and rooted to establish a plantlet. Often the same tissue can be induced to form shoots or embryos by manipulating the components of the culture conditions. In the following sub sections we will discuss organogenesis and embryogenesis in detail. Organogenesis Organogenesis refers to the differentiation of organs such as roots, shoots or flowers. Shoot bud differentiation may occur directly from the explant or from the callus. The stimulus for organogenesis may come from the medium, from the endogenous compounds produced by the cultured tissue or substances carried over from the original explant. Organogenesis is chemically controlled by growth regulators. Skoog while working with tobacco pith callus, observed that the addition of an auxin Indole Acetic Acid (IAA) enhanced formation of roots and suppressed shoot differentiation. He further observed that adenine sulphate, (Cytokinin) reversed the inhibition of auxin and promoted the formation of shoots. You should know that: ADVANCED PHARMACOGNOSY
- 26. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P 1) Organogenesis is contolled by a balance between cytokinin and auxin concentration i.e. it is their relative rather than the absolute concentration that determines the nature of differentiation. 2. A relatively high auxin: Cytokinin ration induces root formation, whereas a high cytokinin: auxin ratio favours shoot bud differentiation. 3. Differential response to exogenously applied growth regulators may be due to differences in the endogenous levels of the hormones within the tissue. Organogenesis is a complex process. Whereas in the cultured tissues of many species organogeiiesis can be demonstrated in this pattern, some plants, notably the monocots, are exceptions. Somatic Embryogenesis The process of embryo development is called embryogenesis. It is not the monopoly of the egg to form an embryo. Any cell of the female gametophyte (Embryo sac) or even of the sporophytic tissues around the embryo sac may give rise to an embryo. Thus we can say that 'The phenomenon of embryogenesis is not necessarily confined to the reproductive cycle". In this subsection we will discuss -,- some examples of "embeos formed in culture", also referred to as "somatic - embryos". The first observation of somatic embryos were made m Dacus Carota. Other plants in which the phenomenon has been studied in some detail are Ranunculus scleratus, citrus and coflea spp. In Rarrunculus scleratus somatic as well as various floral tissues, including anthers proliferated to form callus which, after limited unorganised growth differentiated several embryos. These embryos germinated in situ and a fresh crop of embryos appeared on the surface of the seedling. The embryos were derived from individual epidern~al cells of the hypocotyl Citrus is commonly cited as an example of natural polyembryony Figure 31.3: Guidelines for the production of secondary metabolites from plant organ cultures. 1. Laboratory Design and Development ADVANCED PHARMACOGNOSY
- 27. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P The size of tissue culture lab and the amount and type of equipment used depend upon the nature of the work to be undertaken and the funds available. A standard tissue culture laboratory should provide facilities for: • washing and storage of glassware, plasticware • preparation, sterilization and storage of nutrient media • aseptic manipulation of plant material • maintenance of cultures under controlled temperature, light and humidity • observation of cultures, data collection and photographic facility • acclimatization of in vitro developed plants. The overall design must focus on maintaining aseptic conditions. At least three separate rooms should be available one for washing up, storage and media preparation (the media preparation room); a second room, containing laminar-air-flow or clean air cabinets for dissection of plant tissues and subculturing (dissection room or sterilization room); and the third room to incubate cultures (culture room). This culture room should contain a culture observation table provided with binoculars or stereozoom microscope and an adequate light source. Additionally, a green house facility is required for hardening-off in vitro plantlets. For a commercial set-up, a more elaborate set-up is required. 1.1. Media preparation room The washing area in the media room should be provided with brushes of various sizes and shapes, a large sink, preferably lead-lined to resist acids and alkalis, and running hot and cold water. It should also have large plastic buckets to soak the labware to be washed in detergent, hot-air oven to dry washed labware and a dust-proof cupboard to store them. If the preparation of the medium and washing of the labware are done in the same room, a temporary partition can be constructed between the two areas to guard any interference in the two activities. A continuous supply of water is essential for media preparation and washing of labware. A water distillation unit of around 2 litre/h, a Milli-Q water purification systems needs to be installed. ADVANCED PHARMACOGNOSY
- 28. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P Figure 2.1: A floor plan for plant tissue culture laboratory 1.2. Culture room The room for maintaining cultures should be maintained at temperature 25 ±2°C, controlled by air conditioners and heaters attached to a temperature controller are used. For higher or lower temperature treatments, special incubators with built-in fluorescent light can be used outside the culture room. Cultures are generally grown in diffuse light from cool, white, fluorescent tubes. Lights can be controlled with automatic time clocks. Generally, a 16-hour day and 8-hour nights are used. The culture room requires specially designed shelving to store cultures. Some laboratories have shelves along the walls, others have them fitted onto angle-iron frames placed in a convenient position. Shelves can be made of rigid wire mesh, wood or any building material that can be kept clean and dust-free. Insulation between the shelf lights and the shelf above will ensure an even temperature around the cultures. While flasks, jars and petridishes can be placed directly on the shelf or trays of suitable sizes, culture tubes require some sort of support. Metallic wire racks or polypropylene racks, each with a holding capacity of 18-24 tubes, are suitable for the purpose. 1.3. Dissection room or sterilization room This area should have restricted entry, which is needed to ensure the sterile conditions required for the transfer operations. For sterile transfer operations, the laminar-air-flow cabinets are used. Temperature control is essential in this room as the heat is produced continuously from the flames of burners in the hoods. The room should be constructed in a way to minimize the dust particles and for easy cleaning. Several precautions can be taken including the removal of shoes before entering the area. The laminar horizontal flow sterile transfer cabinets are available in various sizes from many commercial sources. They should be designed with horizontal air flow from the back to the front, and equipped with gas cocks if gas burners are to be used. Electrical outlets are needed for use of electric sterilizers and microscopes, and if weighing is to be done in the hoods. A stainless steel working platform is most durable, easy to keep clean and to prevent the unwanted damage due to accidental fire. Sometimes it is fitted with ADVANCED PHARMACOGNOSY
- 29. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P Ultraviolet light to maintain sterility inside the cabinet. UV light is a source of ozone, which can be mutagenic, therefore, utmost care is to be taken while using this. Although UV light is not necessary, a short exposure time of 3-5 min to cabinet is fine sometimes. Work can be started after 10-15 min of switching on the air flow, and one can work uninterrupted for long hours. A Laminar-air-flow cabinet has small motor to blow air which first passes through a coarse filter, where it loses large particles, and subsequently through a fine filter known as ‘high efficiency particulate air (HEPA). The HEPA filters remove particles larger than 0.3 μm, and the ultraclean air flows through the working area. The velocity of the ultra clean air is about 27 ± 3 m min-1 which is adequate for preventing the contamination of the working area as long as the flow is on. The flow of the air does not in any way hamper the use of a spirit lamp or a Bunsen burner. 1.4. Greenhouse The greenhouse facility is required to grow parent pants and to acclimatize in vitro raised plantlets. The size and facility inside the green house vary with the requirement and depends on the funds available with the laboratory. However, minimum facilities for maintaining humidity by fogging, misting or a fan and pad system, reduced light, cooling system for summers and heating system for winters must be provided. It would be desirable to have a potting room adjacent to this facility. 1.5. Equipments and apparatus 1.4.1. Media preparation area • benches at a height suitable to work while standing • pH meter is used to determine the pH of various media used for tissue culture. pH indicator paper can also be used for the purpose but it is less accurate. The standard media pH is maintained at 5.8. • hot-plate-cum-magnetic stirrer for dissolving chemicals and during media preparation • an autoclave or domestic pressure cooker is crucial instrument for a tissue culture laboratory. High pressure heat is needed to sterilize media, water, labware, forceps, needles etc. Certain spores from fungi and bacteria can only be killed at a temperature of 121°C and 15 pounds per square inch (psi) for 15-20 min. A caution should be taken while opening the door of autoclave and it should be open when the pressure drops to zero. Opening the door immediately can lead to a rapid change in the temperature, resulting in breakage of glassware and steam burning of operator. • plastic carboys for storing distilled water required for media preparation and final washing of labware. • balances near dry corner of the media room. High quality microbalance are required to weigh smallest of the quantities. Additionally a top pan balance is required for less sensitive quantities. • hot-air oven to keep autoclaved medium warm before pouring into vessels. It is also used for the dry heat sterilization of clean glassware like, Petridishes, culture tubes, pipettes etc. Typical sterilizing conditions are 160-170 °C/1hr. • Dish washer for cleaning glass pipettes in running water ADVANCED PHARMACOGNOSY
- 30. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P 1.4.2. Storage area • a deep freezer (-20°C to -80°C) / refrigerator for storage of enzyme solutions, stock solutions plant materials and all temperature-sensitive chemicals. • microwave oven to melt agar solidified media • Upright and inverted light microscope with camera attachment for recording the morphogenic responses from various explants, calli, cells and protoplasts. Inverted microscope gives the clear views of cultures settled at the bottom of Petridishes. 1.4.3. Dissection room • laminar-air-flow cabinet within which tissue culture work can be carried out under sterilized environment • glass bead sterilizer where temperature of beads is raised to 250°C in 15-20 min with 15 s cut off. Here the sterilization of instruments is effecting by pushing them into the beads for 5-7 s. This is much safer compare to the Bunsen burner heating of instruments like, forceps, needles, scalpels etc. • binocular microscope to observe surface details and morphogenic responses of cultures and their possible contamination. • low speed table-top centrifuge to sediment cells or protoplasts 1.4.4. Culture room • air (or heating / cooling system) to maintain 25±2 °C temperature • racks for holding test-tubes • lights to provide diffuse light and to maintain photoperiod • shakers with various sized clamps for different sized flasks to grow cells in liquid medium • thermostat and time clock for lights • wall cabinets for dark incubation of cultures 1.4.5. Other apparatus • beakers (100 mL, 250 mL, 1 L, 5 L) • measuring cylinders (5 mL, 10 mL, 25 mL, 50 mL, 100 mL, 500 mL, 1L, 2 L, 5 L) • graduated pipettes and teats • reagent bottles for storing liquid chemicals and stock solutions (glass or plastic) • culture tubes and flasks (glass or polypropylene or disposable) ADVANCED PHARMACOGNOSY
- 31. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P • plastic baskets • filter membrane, preferably nylon, of sizes 0.22 μm and 0.45 μm, holders and hypodermic syringes (for solutions requiring filter sterilization) • large forceps (blunt and fine points) and scalpels for dissecting and subculturing plant material. • Scalpel handles (no. 3) and blades (no. 11) • Chemicals and reagents for preparing culture media • Disposable gloves and masks. • Micropipettes of maximum volume size 5000 μL, 1000 μL, 500 μL, 250 μL, 100 μL (A) Syringe with filter assembly fitted on conical flask, (B) Disassembled filter assembly Forceps and scalpels for dissection, Micropipettes . ADVANCED PHARMACOGNOSY Tissue culture media 1. Preparation and handling The simplest method of preparing media is to use commercially available, dry, powdered media containing mineral elements and growth regulators. By following the procedure written on the packets, dissolve the powder in distilled or demineralized water (10% less than the final volume of the medium). After adding sugar and other desired supplements like, plant growth regulators, make up the final volume with distilled water, adjust the pH, add agar and then autoclave the medium. An alternative method of media preparation is to prepare a series of concentrated stock solutions which can be combined later as required. For preparing stock solutions and media, use glass-distilled or demineralized water and chemicals of high purity, analytical reagent (AR) grade. 1.1. Composition of widely used tissue culture media Both the media listed in the below tables 2 & 3 can be prepared from stock solutions of: i. Macronutrients: As its name suggests, in plant tissue culture media these components provide the elements which are required in large amounts (concentrations greater than 0.5 mmole l-1 ) by cultured plant cells. Macronutrients are usually considered to be carbon, nitrogen, phosphorous, magnesium, potassium, calcium and sulphur. ii. Micronutrients: It provides the elements that are required in trace amounts (concentrations less than 0.5 mmole l-1 ) for plant growth and development. These include, manganese, copper, cobalt, boron, iron, molybdenum, zinc and iodine.
- 32. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P iii. Iron source: It is considered the most important constituent and required for the formation of several chlorophyll precursors and is a component of ferredoxins (proteins containing iron) which are important oxidation : reduction reagents. iv. Organic supplements (vitamins): Like animals, in plants too vitamins provide nutrition for healthy growth and development. Although plants synthesize many vitamins under natural conditions and, therefore, under in vitro conditions they are supplied from outside to maintain biosynthetic capacity of plant cells in vitro. There are no firm rules as to what vitamins are essential for plant tissues and cell cultures. The only two vitamins that are considered to be essential are myo-inositol and thiamine. Myo-inositol is considered to be vitamin B and has many diverse roles in cellular metabolism and physiology. It is also involved in the biosynthesis of vitamin C. v. Carbon source: This is supplied in the form of carbohydrate. Plant cells and tissues in the culture medium are heterotrophic and are dependent on external source of carbon. Sucrose is the preferred carbon source as it is economical, readily available, relatively stable to autoclaving and readily assimilated by plant cells. During sterilization (by autoclaving) of medium, sucrose gets hydrolyzed to glucose and fructose. Plant cells in culture first utilize glucose and then fructose. Besides sucrose, other carbohydrates such as, lactose, maltose, galactose are also used in culture media but with a very limited success. Table 3.1: The media elements and their functions The steps involved in preparing a medium are summarized below: Add appropriate quantities of various stock solutions, including growth regulators and other special supplements. Make up the final volume of the medium with distilled water. Add and dissolve sucrose. After mixing well, adjust the pH of the medium in the range of 5.5-5.8, using 0.1 N NaOH or 0.1 N HCl (above 6.0 pH gives a fairly hard medium and pH below 5.0 does not allow satisfactory gelling of the agar). Add agar, stir and heat to dissolve. Alternatively, heat in the autoclave at low pressure, or in a microwave oven. Once the agar is dissolved, pour the medium into culture vessels, cap and autoclave at 121°C for 15 to 20 min at 15 pounds per square inch (psi). If using pre-sterilized, non-autoclavable plastic culture vessels, the medium may be autoclaved in flasks or media bottles. After autoclaving, allow the medium to cool to around 60°C before pouring under aseptic conditions. ADVANCED PHARMACOGNOSY
- 33. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P Allow the medium to cool to room temperature. Store in dust-free areas or refrigerate at 7°C (temperature lower than 7°C alter the gel structure of the agar). 1.2. Gelling agents The media listed above are only for liquids, often in plant cell culture a ‘semi-solid' medium is used. To make a semi-solid medium, a gelling agent is added to the liquid medium before autoclaving. Gelling agents are usually polymers that set on cooling after autoclaving. i. Agar: Agar is obtained from red algae- Gelidium amansii . It is a mixture of polysaccharides. It is used as a gelling agent due to the reasons: (a) It does not react with the media constituents (b) It is not digested by plant enzymes and is stable at culture temperature. ii. Agarose: It is obtained by purifying agar to remove the agaropectins. This is required where high gel strength is needed, such as in single cell or protoplast cultures. iii. Gelrite: It is produced by bacterium Pseudomonas elodea . It can be readily prepared in cold solution at room temperature. It sets as a clear gel which assists easy observation of cultures and their possible contamination. Unlike agar, the gel strength of gelrite is unaffected over a wide range of pH. However, few plants show hyperhydricity on gelrite due to freely available water. iv. Gelatin: It is used at a high concentration (10%) with a limited success. This is mainly because gelatin melts at low temperature (25°C) and as a result the gelling property is lost. ADVANCED PHARMACOGNOSY
- 34. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P 1.3. Plant growth regulators In addition to nutrients, four broad classes of growth regulators, such as, auxins, cytokinins, gibberellins and abscisic acid are important in tissue culture. In contrast with animal hormones, the synthesis of a plant growth regulator is often not localized in a specific tissue but may occur in many different tissues. They may be transported and act in distant tissues and often have their action at the site of synthesis. Another property of plant growth regulators is their lack of specificity- each of them influences a wide range of processes. The growth, differentiation, organogenesis and embryogenesis of tissues become feasible only on the addition of one or more of these classes of growth regulators to a medium. In tissue culture, two classes of plant growth regulators, cytokinins and auxins, are of major importance. Others, in particular, gibberellins, ethylene and abscisic acid have been used occasionally. Auxins are found to influence cell elongation, cell division, induction of primary vascular tissue, adventitious root formation, callus formation and fruit growth. The cytokinins promote cell division and axillary shoot proliferation while auxins inhibit the outgrowth of axillary buds. The auxin favours DNA duplication and cytokinins enable the separation of chromosome. Besides, cytokinin in tissue culture media, promote adventitious shoot formation in callus cultures or directly from the explants and, occasionally, inhibition of excessive root formation and are, therefore, left out from rooting media. The ratio of plant growth regulators required for root or shoot induction varies considerably with the tissue and is directly related to the amount of growth regulators present at endogenous levels within the explants. In general, shoots are formed at high cytokinin and low auxin concentrations in the medium, roots at low cytokinin and high auxin concentrations and callus at intermediate concentrations of both plant growth regulators. Commonly used plant growth regulators are listed in Table 4. Stock solutions of growth regulators ADVANCED PHARMACOGNOSY
- 35. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P 1 molar = the molecular weight in g/l 1 mM = the molecular weight in mg/l ppm = parts per million = mg/l 2. Establishing aseptic cultures Plant tissue culture media contain sugar and so support the growth of many microorganisms (bacteria and fungi). When these microorganisms reach a medium, they generally grow much faster than the cultured plant materials. Their growth and toxic metabolites will affect, and may even kill, the tissue cultures. It is, therefore, essential to maintain a completely aseptic environment inside the culture vessels. There are several possible sources of contamination of the medium: • the culture vessel • the medium itself • the explant (plant tissue) • the environment of the transfer area • the instruments used to handle plant material during establishment and subculture • the environment of the culture room. Autoclaving media will eliminate contamination from the culture vessel or the medium. In some cases, substances such as gibberellic acid, abscisic acid (ABA), urea and certain vitamins are thermolabile and break down upon autoclaving. These chemicals can be sterilized by membrane filtration using microfilters of pore size 0.22-0.45 μm which is suitable enough to exclude pathogens. Later the filter sterilized compound can be added to autoclaved medium cooled to around 40°C. To prevent the environment of the culture room from being the source of contamination, keep the culture room as dust- free as possible and remove contaminated cultures from the area as soon as they are detected. Ideally, the culture room should be clean, filtered air which has passed through high efficiency particulate air (HEPA) filters. The transfer area in most laboratories is within a laminar air-flow cabinet. A laminar air-flow cabinet has a small fan which blows air through a coarse filter to remove large dust particles and then through a fine HEPA filter to remove microbes, their spores and other particles larger than 0.3 μm. The velocity of the air coming out of the fine filter is about 27 ± 3 m/min, which keeps airborne microorganisms out of the working area. The working area is swabbed with 70% alcohol (or equivalent) and instruments dipped in 70% alcohol, flamed and cooled before use. Caution : Prolonged contact with alcohol can cause skin irritation, and other health problems can result from the inhalation of fumes. Use ethanol rather than methanol, and surgical gloves when handling. Take care ADVANCED PHARMACOGNOSY
- 36. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P with ultraviolet light as it can permanently damage eyes and promote skin cancer. Laminar flow cabinets equipped with ultraviolet light for surface sterilization should be fitted with safety doors which can be closed when ultraviolet light is used. Plant surfaces carry a wide range of microorganisms. The tissue must be thoroughly surface-sterilized before being placed on the nutrient medium. Discard cultures with fungal or bacterial contamination. Solutions of sodium or calcium hypochlorite are usually effective in disinfecting plant tissues. Placing tissues in a 0.5 to 1% solution of sodium hypochlorite for 10 to 15 minutes will disinfect most tissues. Surface sterilants are toxic to plant tissues. Choose the concentration of the sterilizing agent and the length of time to minimize tissue damage, which shows up as white, bleached areas. Other techniques for surface sterilisation include dipping plant material for a few seconds in 90% ethanol or placing in running water for 30 minutes and 2 hours before disinfection. Caution : Take care with powdered calcium hypochlorite as it is a powerful reducing agent. If calcium hypochlorite is stored moist and the container opened later, it can explode. Store calcium hypochlorite in a sealed container in a dry place. A summary of the six steps commonly involved in establishing and maintaining aseptic plant tissue culture follows. i. Collect pieces of plant material (ex-plants) in a screw-cap bottle. Immerse them in a dilute solution of the disinfectant containing a wetting agent. Replace the lid and store the bottle in the laminar air flow cabinet. Shake the bottle two or three times during the sterilization period. ii. Remove the lid and drain carefully. Thoroughly rinse the plant material in sterilized distilled water and replace the lid. After shaking a few minutes, discard the water. Rinse two or three times more. iii. Transfer the material to a pre-sterilized Petri-dishes or test-tubes. iv. Sterilize the required instruments by dipping them in 70% ethanol and flamed them. Allow to cool. Sterilize the instruments after each time they are used to handle tissue. v. Prepare suitable explants from the surface sterilized material using sterilized instruments (scalpels, needles, forceps, etc.). vi. Quickly remove the lid of the culture vessel, transfer the explants on to the medium, flame the neck of the vessel (only if glass) and replace the lid. If handling aseptic plant materials during routine subculture, omit the first two steps. Plant tissue culture techniques 1. Introduction Plant tissue culture has become popular among horticulturists, plant breeders and industrialists because of its varied practical applications. It is also being applied to study basic aspects of plant growth and development. The discovery of the first cytokinin (kinetin) is based on plant tissue culture research. ADVANCED PHARMACOGNOSY
- 37. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P The earliest application of plant tissue culture was to rescue hybrid embryos (Laibach, 1925, 1929), and the technique became a routine aid with plant breeders to raise rare hybrids, which normally failed due to post-zygotic sexual incompatibility. Currently, the most popular commercial application of plant tissue culture is in clonal propagation of disease-free plants. In vitro clonal propagation, popularly called micropropagation, offers many advantages over the conventional methods of vegetative propagation: (1) many species (e.g. palms, papaya) which are not amenable to in vivo vegetative propagation are being multiplied in tissue cultures, (2) the rate of multiplication in vitro is extremely rapid and can continue round the year, independent of the season. Thus, over a million plants can be produced in a year starting from a small piece of tissue. The enhanced rate of multiplication can considerably reduce the period between the selection of plus trees and raising enough planting material for field trials. In tissue culture, propagation occurs under pathogen and pest-free conditions. An important contribution made through tissue culture is the revelation of the unique property of plant cells, called “cellular totipotency”. The totipotency of plant cells was predicted in 1902 by Haberlandt and the first true plant tissue culture on agar was established. Since then plant tissue culture techniques have greatly evolved. The technique has developed around the concept that a cell has the capacity and ability to develop into a whole organism irrespective of their nature of differentiation and ploidy level. Therefore, it forms the backbone of the modern approach to crop improvement by genetic engineering. The principles involved in plant tissue culture are very simple and primarily an attempt, whereby an explant can be to some extent freed from inter-organ, inter-tissue and inter-cellular interactions and subjected to direct experimental control. Regeneration of plants from cultured cells has many other applications. Plant regeneration from cultured cells is proving to be a rich source of genetic variability, called “somaclonal variation”. Several somaclones have been processed into new cultivars. Regeneration of plants from microspore/pollen provides the most reliable and rapid method to produce haploids, which are extremely valuable in plant breeding and genetics. With haploids, homozygosity can be achieved in a single step, cutting down the breeding period to almost half. This is particularly important for highly heterozygous, long-generation tree species. Pollen raised plants also provide a unique opportunity to screen gametic variation at sporophytic level. This approach has enabled selection of several gametoclones, which could be developed into new cultivars. Even the triploid cells of endosperm are totipotent, which provides a direct and easy approach to regenerate triploid plants difficult to raise in vivo. The entire plant tissue culture techniques can be largely divided into two categories based on to establish a particular objective in the plant species: I. Quantitative Improvement (Micropropagation) Adventitious shoot proliferation (leaves, roots, bulbs, corm, seedling- explants etc.) Nodal segment culture Meristem/Shoot-tip culture Somatic embryogenesis Callus culture ADVANCED PHARMACOGNOSY
- 38. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P II. Qualitative Improvement Anther/ Microspore culture Ovary/ Ovule culture Endosperm culture Cell culture Protoplast culture The above techniques are discussed in detail in subsequent chapters. 2. Micropropagation Growing any part of the plant (explants) like, cells, tissues and organs, in an artificial medium under controlled conditions (aseptic conditions) for obtaining large scale plant propagation is called micropropagation. The basic concept of micropropagation is the plasticity, totipotency, differentiation, dedifferentiation and redifferentiation, which provide the better understanding of the plant cell culture and regeneration. Plants, due to their long life span, have the ability to withhold the extremes of conditions unlike animals. The plasticity allows plants to alter their metabolism, growth and development to best suit their environment. When plant cells and tissues are cultured in vitro , they generally exhibit a very high degree of plasticity, which allows one type of tissue or organ to be initiated from another type. Hence, whole plants can be subsequently regenerated and this regenerated whole plant has the capability to express the total genetic potential of the parent plant. This is unique feature of plant cells and is not seen in animals. Unlike animals, where differentiation is generally irreversible, in plants even highly mature and differentiated cells retain the ability to regress to a meristematic state as long as they have an intact membrane system and a viable nucleus. However, sieve tube elements and xylem elements do not divide any more where the nuclei have started to disintegrate, According to Gautheret (1966) the degree of regression a cell can undergo would depend on the cytological and physiological state of the cell. The meristematic tissues are differentiated into simple or complex tissues called differentiation. Reversion of mature tissues into meristematic state leading to the formation of callus is called dedifferentiation. The ability of callus to develop into shoots or roots or embryoid is called redifferentiation. The inherent potentiality of a plant cell to give rise to entire plant and its capacity is often retained even after the cell has undergone final differentiation in the plant system is described as cellular totipotency. 2.1. Micropropagation vs. conventional method of propagation All living plant cells, irrespective of their nature of specialization and ploidy level, have been shown to regenerate plants via organogenesis or embryogenesis. The latter involves a highly specialized mode of development that normally occurs only inside the seed, under the cover of several layers of parental tissues. Consequently, the observation of developing embryos and their isolation in intact and living conditions for experimental studies have been extremely difficult. In vitro production of embryos from somatic and gametic cells has opened up the possibility of obtaining large numbers of embryos of different stages, enabling investigations on cellular, genetic and physiological control of embryogenesis (induction, pattern formation, organ differentiation and maturation). In vitroexpression of cellular totipotency and other techniques of plant tissue culture have also facilitated and/ or accelerated the traditional methods of plant improvement, propagation and conservation. ADVANCED PHARMACOGNOSY
- 39. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P 2.2. Micropropagation vs. vegetative propagation The vegetative propagation has been conventionally used to raise genetically uniform large scale plants for thousands of years. However, this technique is applicable to only limited number of species. In contrast to this, micropropagation has several advantages which are summarized here: i. The rapid multiplication of species difficult to multiply by conventional vegetative means. The technique permits the production of elite clones of selected plants. ii. The technique is independent of seasonal and geographical constraints. iii. It enable large numbers of plants to be brought to the market place in lesser time which results in faster return on the investment that went into the breeding work. iv. To generate disease-free (particularly virus-free) parental plant stock. v. To raise pure breeding lines by in vitro haploid and triploid plant development in lesser time. vi. It can be utilized to raise new varieties and preservation of germplasm vii. It offers constant production of secondary medicinal metabolites. 2.3. Cell differentiation During in-vitro and in vivo cytodifferentiation (cell differentiation), the main emphasis has been on vascular differentiation, especially tracheary elements (TEs). These can be easily observed by staining and can be scored in macerated preparations of the tissues. Tissue differentiation goes on in a fixed manner and is the characteristic of the species and the organs 2.4. Factors affecting vascular tissue differentiation Vascular differentiation is majorly affected qualitatively and quantitatively by two factors, auxin and sucrose. Cytokinins and gibberellins also play an important role in the process of xylogenesis. Depending upon the characteristics of different species, concentration of phytohormones, sucrose and other salt level varies and accordingly it leads to the vascular tissue differentiation. 3. Micropropagation techniques 3.1. Strategies for propagation in vitro Typical micropropagation system can be broadly divided into five distinct stages (Figure 4.1): The stage zero is the selection of mother plant and preparation of explant. The first stage is the initiation of a sterile culture of the explant in a particular enriched medium for specific species. The second stage includes initiation of cell division from almost any part of the plant system to initiate regeneration or multiplication of shoots or other propagules from the explant. Adventitious shoot proliferation is the most frequently used multiplication technique in micropropagation systems. The culture media and growth conditions used in second stage need to be optimized for maximum rate of multiplication. ADVANCED PHARMACOGNOSY
- 40. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P The third stage is the development of roots on the shoots to produce plantlets. Specialized media may or may not be required to induce roots, depending upon the species. The final or the fourth stage is to produce self-sufficient plants. This stage usually involves a hardening-off process and acclimatization of plants in soil under green-house conditions for later transplanting to the field. Mode of differentiation Regenerants may differentiate either directly from the explants or indirectly via callusing. Dedifferentiation favours unorganized cell growth and the resultant developed callus has meristems randomly distributed in the callus. Most of these meristems, if provided appropriate invitro conditions, would differentiate shoot-buds, roots or embryos. Figure 4.1: Micropropagation stages 4. Trouble shooting • Few explants exude dark colored compounds, like phenols, pigments etc which leach into the medium from the cut ends of the explant. It results in the browning of tissues and the medium as well. The browning of medium is associated with poor culture establishment and low regeneration capacity of the explants. This can be overcome by: i. minimizing the wounding of explants during isolation and surface disinfection to reduce this browning response. ii. washing or incubation of explants for 3-5 hrs in sterile distilled water to remove phenolics responsible for browning of medium or explants. iii. frequent subculture of explants with excision to fresh medium at regular intervals. ADVANCED PHARMACOGNOSY
- 41. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P iv. initial establishment of cultures in liquid medium and later transfer to the semi-solid medium. vi. culture of explants on porous substrate or paper bridges. vi. addition of activated charcoal (AC) or polyvinylpyrrolidone (PVP) for adsorbtion of phenolics. vii. antioxidants like ascorbic acid, citric acid etc. can also be used to prevent browning of tissues in culture. • Appearance of vitrified tissues (hyperhydricity), a physiological disorder occurring in the in vitro cultures due to which the tissues look transparent and fluffy resulting from excessive intake of water. Hyperhydricity can be caused by a high concentration of cytokinin or low concentration of gelling agent or high water retention capacity of explants if the container is tightly closed. • Loss of regeneration ability in long-term cultures due to epigenetic variations (temporary variations) and culture aging, including transition from juvenile to mature stage. Epigenetic variation are phenotypic temporary variations which disappear as soon as the culture conditions are removed. • Genotypic variations are also seen in the cultures, therefore, cytological, biochemical and molecular analyses are required to confirm clonal fidelity of in vitro regenerants. Besides, morphological and physiological testing is also required to remove undesired genetic variability. Plant tissue culture Plant tissue culture is a collection of techniques used to maintain or grow plant cells, tissues or organs under sterile conditions on a nutrient culture medium of known composition. Plant tissue culture is widely used to produce clones of a plant in a method known as micropropagation. Different techniques in plant tissue culture may offer certain advantages over traditional methods of propagation, including: The production of exact copies of plants that produce particularly good flowers, fruits, or have other desirable traits. To quickly produce mature plants. The production of multiples of plants in the absence of seeds or necessary pollinators to produce seeds. The regeneration of whole plants from plant cells that have been genetically modified. The production of plants in sterile containers that allows them to be moved with greatly reduced chances of transmitting diseases, pests, and pathogens. The production of plants from seeds that otherwise have very low chances of germinating and growing, i.e.: orchids and Nepenthes. To clean particular plants of viral and other infections and to quickly multiply these plants as 'cleaned stock' for horticulture and agriculture. Plant tissue culture relies on the fact that many plant cells have the ability to regenerate a whole plant (totipotency). Single cells, plant cells without cell walls (protoplasts), pieces of leaves, stems or roots can often be used to generate a new plant on culture media given the required nutrients and plant hormones. Techniques Modern plant tissue culture is performed under aseptic conditions under HEPA filtered air provided by a laminar flow cabinet. Living plant materials from the environment are naturally contaminated on their surfaces (and sometimes interiors) with microorganisms, so surface sterilization of starting material (explants) in chemical solutions (usually alcohol and sodium or calcium hypochlorite or mercuric chloride[1] is required. Mercuric chloride is seldom used as a plant sterilant today, unless other sterilizing ADVANCED PHARMACOGNOSY
- 42. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P agents are found to be ineffective, as it is dangerous to use, and is difficult to dispose of. Explants are then usually placed on the surface of a solid culture medium, but are sometimes placed directly into a liquid medium, particularly when cell suspension cultures are desired. Solid and liquid media are generally composed of inorganic salts plus a few organic nutrients, vitamins and plant hormones. Solid media are prepared from liquid media with the addition of a gelling agent, usually purified agar. The composition of the medium, particularly the plant hormones and the nitrogen source (nitrate versus ammonium salts or amino acids) have profound effects on the morphology of the tissues that grow from the initial explant. For example, an excess of auxin will often result in a proliferation of roots, while an excess of cytokinin may yield shoots. A balance of both auxin and cytokinin will often produce an unorganised growth of cells, or callus, but the morphology of the outgrowth will depend on the plant species as well as the medium composition. As cultures grow, pieces are typically sliced off and transferred to new media (subcultured) to allow for growth or to alter the morphology of the culture. The skill and experience of the tissue culturist are important in judging which pieces to culture and which to discard. As shoots emerge from a culture, they may be sliced off and rooted with auxin to produce plantlets which, when mature, can be transferred to potting soil for further growth in the greenhouse as normal plants. Choice of explant The tissue obtained from a plant to be cultured is called an explant based on work with certain model systems particularly tobacco it has often been claimed that a totipotent explant can be grown from any part of the plant and may include portions of shoots, leaves, stems, flowers, roots and single, undifferentiated cells.,[citation needed] however this has not been true for all plants.[3] In many species explants of various organs vary in their rates of growth and regeneration, while some do not grow at all. The choice of explant material also determines if the plantlets developed via tissue culture are haploid or diploid. Also the risk of microbial contamination is increased with inappropriate explants. The specific differences in the regeneration potential of different organs and explants have various explanations. The significant factors include differences in the stage of the cells in the cell cycle, the availability of or ability to transport endogenous growth regulators, and the metabolic capabilities of the cells. The most commonly used tissue explants are the meristematic ends of the plants like the stem tip, auxiliary bud tip and root tip. These tissues have high rates of cell division and either concentrate or produce required growth regulating substances including auxins and cytokinins. The pathways through which whole plants are regenerated from cells and tissues or explants such as meristems broadly fall into three types: 1. The method in which explants that include a meristem (viz. the shoot tips or nodes) are grown on appropriate media supplemented with plant growth regulators to induce proliferation of multiple shoots, followed by rooting of the excised shoots to regenerate whole plants, 2. The method in which totipotency of cells is realized in the form of de novo organogenesis, either directly in the form of induction of shoot meristems on the explants or indirectly via a callus (unorganised mass of cells resulting from proliferation of cells of the explant) and plants are regenerated through induction of roots on the resultant shoots, 3. Somatic embryogenesis, in which asexual adventive embryos (comparable to zygotic embryos in their structure and development) are induced directly on explants or indirectly through a callus phase. The first method involving the meristems and induction of multiple shoots is the preferred method for the micropropagation industry since the risks of somaclonal variation (genetic variation induced in tissue culture) are minimal when compared to the other two methods. Somatic embryogenesis is a method that has ADVANCED PHARMACOGNOSY
- 43. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P the potential to be several times higher in multiplication rates and is amenable to handling in liquid culture systems like bioreactors. Some explants, like the root tip, are hard to isolate and are contaminated with soil microflora that become problematic during the tissue culture process. Certain soil microflora can form tight associations with the root systems, or even grow within the root. Soil particles bound to roots are difficult to remove without injury to the roots that then allows microbial attack. These associated microflora will generally overgrow the tissue culture medium before there is significant growth of plant tissue. Aerial (above soil) explants are also rich in undesirable microflora. However, they are more easily removed from the explant by gentle rinsing, and the remainder usually can be killed by surface sterilization. Most of the surface microflora do not form tight associations with the plant tissue. Such associations can usually be found by visual inspection as a mosaic, de-colorization or localized necrosis on the surface of the explant. An alternative for obtaining uncontaminated explants is to take explants from seedlings which are aseptically grown from surface-sterilized seeds. The hard surface of the seed is less permeable to penetration of harsh surface sterilizing agents, such as hypochlorite, so the acceptable conditions of sterilization used for seeds can be much more stringent than for vegetative tissues. Tissue cultured plants are clones. If the original mother plant used to produce the first explants is susceptible to a pathogen or environmental condition, the entire crop would be susceptible to the same problem. Conversely, any positive traits would remain within the line also. Applications Plant tissue culture is used widely in the plant sciences, forestry, and in horticulture. Applications include: The commercial production of plants used as potting, landscape, and florist subjects, which uses meristem and shoot culture to produce large numbers of identical individuals. To conserve rare or endangered plant species.[4] A plant breeder may use tissue culture to screen cells rather than plants for advantageous characters, e.g. herbicide resistance/tolerance. Large-scale growth of plant cells in liquid culture in bioreactors for production of valuable compounds, like plant-derived secondary metabolites and recombinant proteins used as biopharmaceuticals.[5] To cross distantly related species by protoplast fusion and regeneration of the novel hybrid. To cross-pollinate distantly related species and then tissue culture the resulting embryo which would otherwise normally die (Embryo Rescue). For production of doubled monoploid (dihaploid) plants from haploid cultures to achieve homozygous lines more rapidly in breeding programmes, usually by treatment with colchicine which causes doubling of the chromosome number. As a tissue for transformation, followed by either short-term testing of genetic constructs or regeneration of transgenic plants. Certain techniques such as meristem tip culture can be used to produce clean plant material from virused stock, such as potatoes and many species of soft fruit. Production of identical sterile hybrid species can be obtained. ADVANCED PHARMACOGNOSY
- 44. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P Callus Culture: When the cells divide into an undifferentiated mass it is called as callus. Any part of a plant can be used to produce the calli. It may be a stem, leaf, meristem or any other part. It is used to produce variations among the plantlets. Callus formation is induced from plant tissues after surface sterilization and plating onto in vitro tissue culture medium. Plant growth regulators, such as auxins, cytokinins, andgibberellins, are supplemented into the medium to initiate callus formation or somatic embryogenesis. Plant callus is usually derived from somatic tissues. The tissues used to initiate callus formation depends on plant species and which tissues are available for explant culture. The cells that give rise to callus and somatic embryos usually undergo rapid division or are partially undifferentiated such as meristematic tissue. In alfalfa,Medicago truncatula, however callus and somatic embryos are derived from mesophyll cells that undergo dedifferentiation.[17] Plant hormones are used to initiate callus growth. Specific auxin to cytokinin ratios in plant tissue culture medium give rise to an unorganized growing and dividing mass of callus cells. Callus cultures are often broadly classified as being either compact or friable. Friable calluses fall apart easily, and can be used to generate cell suspension cultures. Callus can directly undergo direct organogenesis and/or embryogenesis where the cells will form an entirely new plant. Callus induction and tissue culture A callus cell culture is usually sustained on gel medium. Callus induction medium consists of agar and a mixture of macronutrients and micronutrients for the given cell type. There are several types of basal salt mixtures used in plant tissue culture, but most notably modified Murashige and Skoog medium,[13] White's medium,[14] and woody plant medium.[15] Vitamins are also provided to enhance growth such as Gamborg B5 vitamins.[16] For plant cells, enrichment with nitrogen, phosphorus, andpotassium is especially important. Callus cells deaths Callus can brown and die during culture, but the causes for callus browning are not well understood. In Jatropha curcas callus cells, small organized callus cells became disorganized and varied in size after ADVANCED PHARMACOGNOSY
- 45. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P browning occurred.[18] Browning has also been associated with oxidation and phenolic compounds in both explant tissues and explant secretions. Suspension culture: The callus produced from the explants are grown on nutrient solutions (that are semi solid) for a period of time and they are induced to produce plants with new traits. A callus crumbles into smaller clumps and single cells in liqu~d~medium by gentle agitation (100-120rPM) on a shaker. Shaking the cultures also helps to aerate the cells. Such suspension cultures however rarely comprise single cells alone because cells tend to aggregate in clusters of 2-100. Suspension cultures can be maintained indefinitely by inoculations of known aliquot5 of cells to a fresh medium. This process is termed as "batch cultures". Alternatively, the medium is replenished at regu lar intervals. This process is termed as "continuous culture". In the continuous culture process at the time of replenishing the medium, cells are also harvested (open continuous system) or the biomass is allowed t~*increase (close continuous system). Suspension cultures are useful in studying problems related to cell biology including cell cycle and production of secondary metabolites like alkaloids, steroids, glycosides, napthaquinones, flavones etc. which find medicinal and industrial application. Pharmaceutical industries use large bioreactors for suspension cultures to obtain valuable bioorganic compounds. A bioreactor is a vessel of glass or steel in which cells are cultured aseptically and culture conditions are closely monitored. This results in higher yield of metabolites. In a bioreactor there is provision for adding fresh medium, for harvesting cells, for the aeration of products, for mixing and sampling, for controlling pH, 02 content and temperature Plant cells are immobilised in alginate, agarose, polyacrylamide beads. Immobilisation of cells enables i) re-use of biomass by rotation of cells ii) separation of cells from the medium and iii) leaching of metabolites in it . Immobilised cells are cultured in column reactors. Column reactors are of different types with different agitation and flow systems. Such reactors may be i) stirred tank type ii) air lift type iii) bubble column type and iv) rotating drum type. 11.3.2 Single Cell Culture This is an important invitro technique which enables the cloning of selected cells. Single cells can be obtained directly from plant organs by treatment with enzymes that dissolve middle lamellae. The separate cells can sieve into liquid medium to start a suspension culture. The most widely used technique for single cell culture is the Bergmann's method of Cell Plating and. Microchamber technique. Bergmann's Method of Cell Plating: In this method free cells are suspended in a liquid medium at a density twice the Plant Tissue And Organ finally desired plating density. Melted agarcontaining medium of otherwise the Culture same composition as the liquid medium is maintained at 35Oc in water bath. Equal volumes of the two media are mixed and rapidly spread out in petri dishes in such a manner that the cells are evenly distributed and fixed in a thin layer (about 1 mm thick) of the medium after it has cooled and solidified. The dishes are sealed with parafilm. The cells to be followed are marked on the outside of the plate and before the colonies derived from individual cells grow large enough to merge with each other. They are transferred to.separate plates. (Fig. 11.3). Another popular method for single cell culture is the microchamber technique, developed by Jones et al. (1960). In this method mechanically isolated single cells are cultured in separate droplets of liquid medium. While Jones et al. used sterile microslides and three coverglasses to make microchamber, it is now possible to buy pre-sterilised plastic plates with several microwells (Cuprak dishes). Individual cells are cultured in separate wells each containing 0.25 ml of the liquid medium. The culture requirement of single cells increases with decrease in the plating cell density, and the cell cultured in complete isolation require a very complex culture medium. A simple medium conditioned by growing cell suspension for some time rlso fulfils the requirements of single cell culture at low density ADVANCED PHARMACOGNOSY
- 46. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P Clonal Propagation Most cultivars of ornamental and fruit species and forest trees are highly heterozygous. Consequently, their seed progeny is not true-to-type. To preserve the unique characters of selected cultivars of horticultural plants nurserymen practise vegetative propagation, using stem, leaf or root cuttings or propagules such as tubers, corms, bulbs or bulbils. For plants which do not set seeds, such as edible bananas,grapes, citrus, petunia, rose and chrysanthemum, vegetative propagation is the only means of multiplication. A population of plants derived from a single individual by vegetative propagation is genetically uniform and is called a clone. The conventional methods of clonal propagation are slow and often not applicable. For example, the only in-vivo method for clonal multiplication of cultivated orchids, which are complex hybrids,is "back-bulb" propagation. It involves separating the oldest pseudobulbil to force the development of dormant buds. This process allows, at best, doubling the plant number every year. Moreover, Diagrammatic summary of steps involved in aseptic multiplication of plants. Shoot multiplication is achieved through enhanced axillary branching adventitious budding from explants directly or after callusing The shoots are rooted individually in a me- dium containing an auxin. The plantlets so obtained are transferred to well drained potting mix. After maintaining them under high humidity for3-4 weeks the plants are transferred to ordinary glasshouse or field conditions Plant multiplication involving a callus phase may occur via shoot bud differentiation or somatic embryogenesis. In the latter case the rooting step is eliminated as the embryos possess a pre-formed root primordiurn. monopodial orchids do not form pseudobulbils and, therefore, cannot be clonally multiplied. In 1960, a French scientist, G.More1, described an in-vitro method for rapid clonal multiplication of orchids. This revolutionised the orchid industry, and today tissue culture is the only economically feasible method for clonal multiplication of orchids and is being widely used. In-vitro clonal propagation, popularly called Micropropagation has been extended to a large number of species other than orchids and is being practised on commercial scale for numerous ornamental and fruit bearing plant and some forest trees. After the initiation of aseptic cultures micropropagation generally involves three steps: Shoot multiplication, rooting and transplantation. Shoot Multiplication: This is the most important step with respect to the rate of propagation and genetic uniformity of the product. The most reliable and, therefore, themost popular method of shoot multiplication is forced proliferation of axillary shoots. For this, cultures are initiated from apical or nodal cuttings carrying one or more vegetative buds. In the presence of a cytokinin alone or in combination with a low concentration of an auxin, such as IAA or NAA, the pre-existing buds grow and produce 4-6 shoots (sometimes up to 30-40 shoots) within 3-4 weeks. By periodic removal of individual shoots and planting them on fresh medium of the original composition, the shoot multiplication cycle can be repeated almost indefinitely, and a stock of large number of shoots built up in a short period of time. Treatments with PGRs as described above can also help in a rapid build up of shoots by inducing adventitious buds by the explant directly or after callusing. Somatic embryogenesis, which generally occurs after callusing of the explant, is another method of micro propagation. Somatic embryogenesis is not only fast, but may also allow partial automation of micropropagation and the propagules so produced (somatic embryos) bear both, shoot and root meristems. However, adventitive differentiation of shoots or somatic embryos, especially from callus tissue, has the risk of genetic variability in the progeny. Such variation, that develops in tissue culture called "somaclonal variation" is not desirable for micropropagation but is being exploited as a novel source of useful variations for crop improvement. Rooting: ADVANCED PHARMACOGNOSY
- 47. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P Shoots produced through axillary branching or adventitious differentiation are rooted in-vitro on a medium containing a suitable auxin, such as IAA, NAA or IBA. Alternatively, where possible, the shoots are treated with auxin and directly planted in potting mixture for in-vivo rooting. Transplantation: The shoots or plantlets multiplied on a medium containing organic nutrients, show poor photosynthetic capability. Moreover, in these plants mechanisms to prevent * loss of water from leaves are poorly developed. Therefore, they require gradual acclimatization to the field conditions. In practice, the plants are maintained under high humidity (80-90%) for 10-15 days after they are removed from culture vessels. During the next few weeks the hu~idity around the plants is gradually lowered, before they are transferred to naturtil conditions. The special merits of micropropagation are: i) it considerably increases the rate of multiplication 2) high rate of multiplication can be maintained throughout the year, 3) the multiplied plants are maintained in disease-free conditions 4) being free from microbes and insects valuable genotypes of exotic plants can be multiplied for export purpose, and 5) small size of the propagules and their ability to proliferate in a soil-less environment facilitates their convenient storage, handling and rapid transfer by air across international quarantine baniers. Uses of plant tissue culture Plant tissue culture now has direct commercial applications as well as value in basic research into cell biology, genetics and biochemistry. The techniques include culture of cells, anthers, ovules and embryos on experimental to industrial scales, protoplast isolation and fusion, cell selection and meristem and bud culture. Applications include: micropropagation using meristem and shoot culture to produce large numbers of identical individuals screening programmes of cells, rather than plants for advantageous characters large-scale growth of plant cells in liquid culture as a source of secondary products crossing distantly related species by protoplast fusion and regeneration of the novel hybrid production of dihaploid plants from haploid cultures to achieve homozygous lines more rapidly in breeding programmes as a tissue for transformation, followed by either short-term testing of genetic constructs or regeneration of transgenic plants removal of viruses by propagation from meristematic tissues IMPORTANCE AND HISTORICAL VIEW OF PLANT TISSUE CULTURE Objective To begin with, one should know the importance of plant tissue culture in theimprovement of useful crop plants and also the ways in which it has helped mankind. Planttissue culture forms an integral part of any plant biotechnology activity. It offers an alternativeto conventional vegetative propagation. But, tissue culture requires attention-to-detail andunless practiced as art and science, the entire process is ratherunforgiving. The various objectives achievable or achieved by plant tissue culture may besummarized as under: a. Crop Improvement As you all understand that for any crop improvement, conventional breeding methodsare employed which involve six to seven generations of selfing and crossing- over to obtain apure line. With plant tissue culture techniques, production of haploids through distant crossesor using pollen, anther or ovary culture, followed by chromosome doubling, reduces this timeto two generations. b. Micropropagation ADVANCED PHARMACOGNOSY
- 48. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P Plant tissue culture techniques have also helped in large- scale production of plantsthrough micropropagation or clonal propagation of plant species. Small amounts of tissue canbe used to raise hundreds or thousands of plants in a continuous process. This is beingutilized by industries in India for commercial production of mainly ornamental plants likeorchids and fruit trees, e.g., banana. Using this method, millions of genetically identical plantscan be obtained from a single bud. This method has, therefore, become an alternative tovegetative propagation. Shoot tip propagation is exploited intensively in horticulture and thenurseries for rapid clonal propagation of many dicots, monocots and gymnosperms. c. Genetic Transformation Tissue culture, in combination with genetic engineering is very useful in gene transfers.For example, the transfer of a useful bacterial gene say, cry (crystal protein) gene from Bacillus thuringiensis , into a plant cell and, ultimately, regeneration of whole plants containing andexpressing this gene (transgenic plants) can be achieved. d. Production of Pathogen-free Plants Eradication of virus has been an outstanding contribution of tissue culture technology.It was found that even in infected plants the cells of shoot tips are either free of virus or carry anegligible amount of the pathogen. Such shoot tips are culturedin a suitable culture medium to obtain virus- free plants. This technique is economical andused very frequently in horticulture, production of virus- free ornamentals etc. e. Production of Secondary Metabolites Cultured plant cells are also known to produce biochemicals [secondary metabolites]like, alkaloids, terpenoids, phenyl propanoids etc. of interest. The technology is now availableto the industry. The commercial production of ‘shikonin’[a naphthoquinone] from cell cultures of Lithospermum erythrorhizon, has been particularly encouraging Applications of immobilized enzymes The first industrial use of an immobilized enzyme is amino acid acylase by Tanabe Seiyaku Company, Japan, for the resolution of recemic mixtures of chemically synthesized amino acids. Amino acid acylase catalyses the deacetylation of the L form of the N-acetyl amino acids leaving unaltered the N-acetyl-d amino acid, that can be easily separated, racemized and recycled. Some of the immobilized preparations used for this purpose include enzyme immobilized by ionic binding to DEAE-sephadex and the enzyme entrapped as microdroplets of its aqueous solution into fibres of cellulose triacetate by means of fibre wet spinning developed by Snam Progetti. Rohm GmbH have immobilized this enzyme on macroporous beads made of flexiglass-like material By far, the most important application of immobilized enzymes in industry is for the conversion of glucose syrups to high fructose syrups by the enzyme glucose isomerase95. Some of the commercial preparations have been listed. It is evident that most of the commercial preparations use either the adsorption or the cross-linking technique. Application of glucose isomerase technology has gained considerable importance, especially in nontropical countries that have abundant starch raw material. Unlike these countries, in tropical countries like India, where sugarcane cultivation is abundant, the high fructose syrups can be obtained by a simpler process of hydrolysis of sucrose using invertase. Compared to sucrose, invert sugar has a higher humectancy, higher solubility and osmotic pressure. Historically, invertase is perhaps the first reported enzyme in an immobilized form96. A large number of immobilized invertase systems have been patented97. The possible use of whole cells of yeast as a source of invertase was demonstrated by D’Souza and Nadkarni as early as 1978. A systematic study has been carried out in our laboratory for the preparation of invert sugar using immobilized invertase or the whole cells of yeast. These comprehensive studies carried ADVANCED PHARMACOGNOSY
- 49. T.B.EKNATH BABU (T.B.E.K.B) STUDENT AT A.K.C.P out on various aspects in our laboratory of utilizing immobilized whole-yeast have resulted in an industrial process for the production of invert sugar. L-aspartic acid is widely used in medicines and as a food additive. The enzyme aspartase catalyses a one-step stereospecific addition of ammonia to the double bond of fumaric acid. The enzymes have been immobilized using the whole cells of Escherichia coli. This is considered as the first industrial application of an immobilized microbial cell. The initial process made use of polyacrylamide entrapment which was later substituted with the carragenan treated with glutaraldehyde and hexamethylenediamine. Kyowa Hakko Kogyo Co. uses Duolite A7, a phenolformaldehyde resin, for adsorbing aspartase used in their continuous process99. Other firms include Mitsubishi Petrochemical Co.100 and Purification Engineering Inc101. Some of the firms, specially in Japan like Tanabe Seiyaku and Kyowa Hakko, have used the immobilized fumarase for the production of malic acid (for pharmaceutical use)94. These processes make use of immobilized nonviable cells of Brevibacterium ammoniagenes or B. flavus as a source of fumarase. Malic acid is becoming of greater market interest as food acidulant in competition with citric acid. Studies from our laboratory have shown the possibility of using immobilized mitochondria as a source of fumarase6. One of the major applications of immobilized biocatalysts in dairy industry is in the preparation of lactose-hydrolysed milk and whey, using b -galactosidase. A large population of lactose intolerants can consume lactose-hydrolysed milk. This is of great significance in a country like India where lactose intolerance is quite prevalent102. Lactose hydrolysis also enhances the sweetness and solubility of the sugars, and can find future potentials in preparation of a variety of dairy products. Lactose-hydrolysed whey may be used as a component of whey-based beverages, leavening agents, feed stuffs, or may be fermented to produce ethanol and yeast, thus converting an inexpensive byproduct into a highly nutritious, good quality food ingredient99. The first company to commercially hydrolyse lactose in milk by immobilized lactase was Centrale del Latte of Milan, Italy, utilizing the Snamprogetti technology. The process makes use of a neutral lactase from yeast entrapped in synthetic fibres103. Specialist Dairy Ingredients, a joint venture between the Milk Marketing Board of England and Wales and Corning, had set up an immobilized b -galctosidase plant in North Wales for the production of lactose-hydrolysed whey. Unlike the milk, the acidic b -galactosidase of fungal origin has been used for this purpose31. Some of the commercial b -galactosidase systems have been summarized in Table 3. An immobilized preparation obtained by cross-linking b -galactosidase in hen egg white (lyophilized dry powder) has been used in our laboratory for the hydrolysis of lactose47. A major problem in the large-scale continuous processing of milk using immobilized enzyme is the microbial contamination which has necessitated the introduction of intermittent sanitation steps. A co-immobilizate obtained by binding of glucose oxidase on the microbial cell wall using Con A has been used to minimize the bacterial contamination during the continuous hydrolysis of lactose by the initiation of the natural lacto-peroxidase system in milk88. A novel technique for the removal of lactose by heterogeneous fermentation of the milk using immobilized viable cells of K. fragilis has also been developed10. One of the major applications of immobilized enzymes in pharmaceutical industry is the production of 6- aminopenicillanic acid (6-APA) by the deacylation of the side chain in either penicillin G or V, using penicillin acylase (penicillin amidase)104. More than 50% of 6-APA produced today is enzymatically using the immobilized route. One of the major reasons for its success is in obtaining a purer product, thereby minimizing the purification costs. The first setting up of industrial process for the production of 6-APA was in 1970s simultaneously by Squibb (USA), Astra (Sweden) and Riga Biochemical Plant (USSR). Currently, most of the pharmaceutical giants make use of this technology. A number of immobilized systems have been patented or commercially produced for penicillin acylase which make use of a variety of techniques either using the isolated enzyme or the whole cells100,105,106. This is also one of the major applications of the immobilized enzyme technology in India. Similar approach has also been used for the production of 7- aminodeacetoxy-cephalosporanic acid, an intermediate in the production of semisynthetic cephalosporins. ADVANCED PHARMACOGNOSY


Comments
Post a Comment